March 28th, 2022
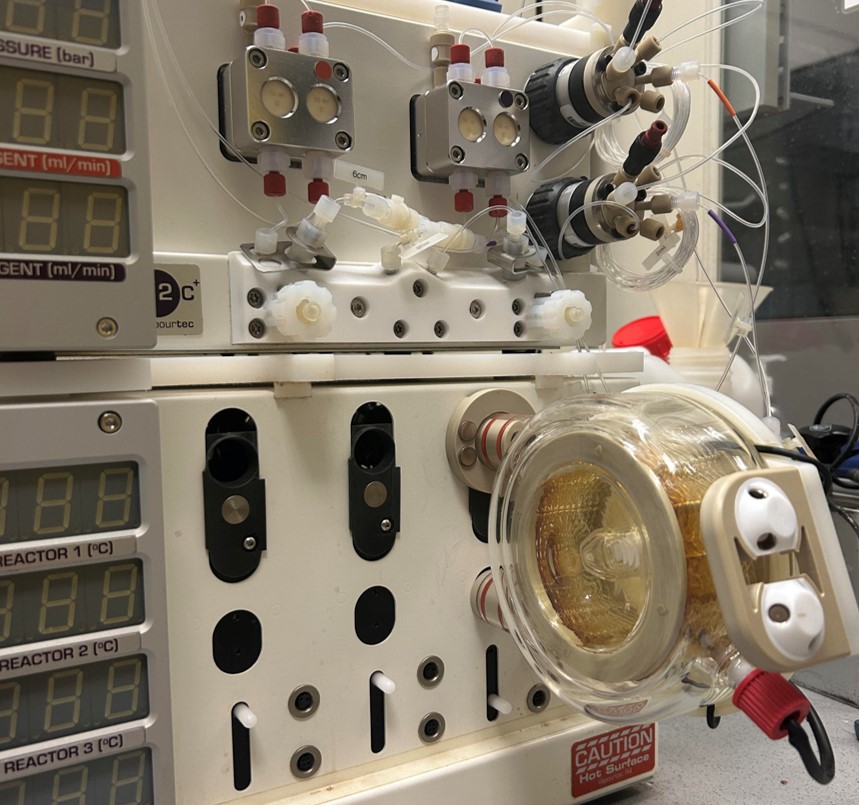
Enantia is proud to announce the commission of new dedicated facilities to work with HPAPIs in solid forms R&D projects.
High potency active pharmaceutical ingredients (HPAPIs) exert their activity at a very low dosage. Though the definition of a HPAPI is yet to be harmonized[1], HPAPIs can be defined based on the therapeutic dose as a pharmacologically-active ingredient with biological activity at approximately 15 μg/kg of body weight or below in humans, or a daily dose of 1 mg/day or below[2].
The global market of HPAPIs is forecasted to grow from 21.2 USD billions in 2021 to 32.17 USD billions in 2026 at an annual increase of 8.7%[3]. Within this category of compounds, the generic drug segment is the one expected to be the fastest growing[4]. Some of the factors attributing to its growth are patent expiry of HPAPI branded drugs and the rising number of patients in developing economies.
Therefore, undertaking a solid form screening to develop a new, non-infringing alternative solid form of a HPAPI can be a highly convenient strategy when aiming at developing new generics, as well as to extend IP rights for new chemical entities (NCE).
Hence, screening for new cocrystals, polymorphs, solvates and salts is a powerful tool that allows to competitively market new forms of a drug. In this sense, FDA[5] and EMA’s[6] regulatory guidelines have set a clear and simple path to the filing of both NDA and ANDA using cocrystals as the solid form of the API.
Enantia’s new facilities allow working with HPAPIs having Operational Exposure Limits (OEL) down to 100 ng/m3, which comprises the overwhelming majority of HPAPIs. Moreover, an additional new X-ray powder diffractometer, working both in transmission and reflection mode, has been installed specifically for HPAPIs analysis.
This means that Enantia also offers for high potency APIs its expertise in:
Moreover, Enantia plans to soon extend all the necessary containment equipment and procedures to the Chemical R&D and Medicinal Chemistry departments in order to execute projects also involving HPAPIs.
If you want to know more about solid state R&D services for HPAPIs keep reading here.
_________________________________________________________
[1] Walsh, Andrew & Barle, Ester. (2017). Are high potency active pharmaceutical ingredients (HPAPI) also high risks for cross-contamination? Chemistry Today. 35.
[2] NTP1104. Industria farmacéutica: clasificación de principios activos en categorías. Actualiza NTP798.
[3] Global HPAPI Market Size, Share, Trends, COVID-19 Impact & Growth Analysis Report – Segmented By Type (Innovative HPAPI’s & Generic HPAPI’s), Synthesis (Biotech HPAPI’s & Synthetic HPAPI’s), Therapeutic Application & Region – Industry Forecast (2022 to 2027), January, 2022, ID: 2903, Pages: 175.
[4] High Potency Active Pharmaceutical Ingredients Market Size, Share & Trends Analysis Report By Product (Synthetic, Biotech), By Manufacturer Type (In-house, Outsourced), By Drug Type, By Application, By Region, And Segment Forecasts, 2021 – 2028. Published Date: Sep, 2021, Report ID: 978-1-68038-563-2, Number of Pages: 140.
[5] Regulatory Classification of Pharmaceutical Co-Crystals Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February 2018 Pharmaceutical Quality/CMC Revision 1.
[6] Reflection paper on the use of cocrystals of active substances in medicinal products EMA/CHMP/CVMP/QWP/284008/2015.